Highlights
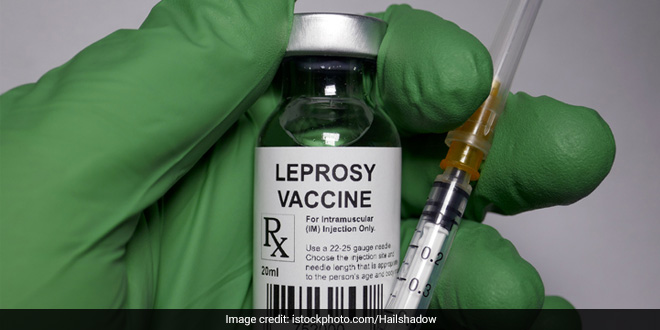
- CSIR is testing Mycobacterium w, a vaccine for leprosy to treat COVID-19
- CSIR has sought approval of Drug Controller General of India to test Mw
- CSIR will release the results of the trial in a couple of months
New Delhi: Council of Scientific and Industrial Research (CSIR) Director-General Dr Shekhar C Mande on Sunday (April 19) said that the institute is currently testing Mycobacterium w (Mw), which is a vaccine for leprosy, to treat COVID-19. “The CSIR is concentrating on immune-boosting vaccines and hence Mw is being tested. We have taken approval of Drug Controller General of India (DCGI) to test Mw for a vaccine of coronavirus,” Mr Mande told ANI.
Also Read: How Long Will The World Have To Wait For A Vaccine Against COVID-19? Experts Answer
Speaking about the Mw vaccine, he said, “The Mw is usually used as a vaccine for leprosy. It is a micro bacterium species and we will be using a strain of it for an immune-boosting vaccine.”
He further said that the CSIR has tied up with several hospitals and they are in the process of recruiting patients for clinical trials.
The results of our trial will be out in a couple of months. If we find that the results are encouraging then we will increase the number of patients, he said.
Also Read: Vaccine Will Be Effective Even If Coronavirus Mutates: ICMR
He said hydroxychloroquine is under advanced trial stages and as per initial indications, it seems to be working on people who are continuously exposed to coronavirus patients.
The real efficacy of the drug will be known only after these trials are over, he added.
On COVID-19 testing kits, the CSIR chief said that in 48 hours two new rapid testing kits have been developed – one by the institute and the other by Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST) in Thiruvananthapuram.
While the kit developed by SCTIMST is RT lamp for testing COVID-19, the CSIR-Institute of Genomics and Integrative Biology has developed a paper-based kit. Both the kits produce accurate results in 30 minutes, Mr Mande said.
Also Read: ICMR Directs All States To Follow Key Protocols For Rapid Testing Of COVID-19
[corona_data_new]