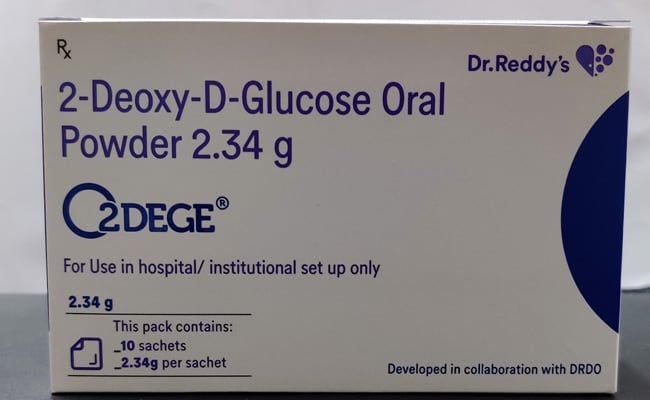
New Delhi: Defence Research and Development Organisation’s (DRDO) new COVID-19 drug – 2-DG has been recently granted permission for as an adjuvant therapy (treatment given in addition to the primary treatment) in moderate to severe COVID patients by the Drugs Controller General of India (DCGI).
Here’s a quick lowdown on this new drug and how it helps in the COVID-19 treatment and what is the opinion of the experts:
DRDO’s Anti-COVID Drug – 2-DG
Basically 2-DG stands for 2-deoxy-D glucose, which is essentially a modified glucose, the type that so far has been used in therapeutic treatments as anti-cancer and anti-viral agent. Now, Defence Research and Development Organisation (DRDO) has said that it should be only used as an additional treatment in moderate to severe COVID patients and not the mild ones.
This drug has been jointly developed by Institute of Nuclear Medicine and Allied Sciences (INMAS), a lab of the Defence Research and Development Organisation (DRDO), in collaboration with Dr Reddy’s Laboratories (DRL), Hyderabad.
The company claims that the drug can be produced easily in huge quantities as well.
The drug essentially comes in a powder form in a sachet and is to be taken twice a day by dissolving it in water, for at least a week.
But how does the DRODO drug help the COVID-19 patients?
The claims so far is that it leads to faster recovery in COVID patients who are hospitalised. It also claims that it reduces the dependence ON oxygen supplement in patientS and works against all the variants of the virus.
Also Read: Dos And Don’ts Of Using Steroids In COVID-19 Treatment
What Is The Technology Behind DRDO’s 2-DG COVID Drug?
For the virus to multiply fast in the body it needs glucose for energy. When the virus feeds on this modified glucose for energy, the claim is that it will get arrested. Which means it will stop from multiplying at that same rate.
What is The Evidence On The Effectiveness Of DRDO’s 2-DG COVID Drug?
The initial clinical data found that the molecule works effectively against SARS-COV2, then DRDO and DRL conducted phase 2 clinical trials on 110 patients. Phase 3 trials were carried out on 220 patients admitted. And at the end of the trials, they concluded that the patients became free from the supplemental oxygen by Day 3.
Also Read: COVID-19 Makes It Harder For People To Access Basic Health Services
Experts View On DRDO’s 2-DG COVID Drug
Talking about the drug and why it was given an emergency approval in the country, Dr Sudhir Chandna, Additional Director Institute of Nuclear Medicine and Allied Sciences, DRDO said,
The data that has been submitted to the drug controller has undergone thorough scrutiny and the peer review publication of the data is in process and will be done very shortly. The one reason why we went for an emergency approval is that we saw that our drug had some indication that really seemed to help COVID patients and their oxygen dependency. Data from all our clinical trials have suggested that the use of drug in COVID patients reduces the oxygen dependency and can save lives.
Highlighting why the number of patients in the clinical trials were this low, Dr Sudhir Chandna said,
Well, the number 220 was not given by us, when clinical trials happen, they happened with full protocol. Initially we were given just a few of the patients to show the proof of confirmation for the dose and if it is safe and effective. And once that was done, the drug controller gave us the permission to carry on with the phase 2 of the trials with dose ranging, in which we increase the dosage of the drugs. So, when the trial was concluded and when the data was presented to the drug controller, it was so distinctive and the proof of its effectiveness was solid that in the phase three trial we were given relatively less people as a part of the trials under the power analysis guidelines. So, in phase three we got the approval for 220 patients and that’s how the trials were carried out. However, if our results were not as distinctive and as good, then in Phase 3, the number of patients would have increased.
On the other hand, Dr Sumit Ray, Head, Critical Care, Holy Family said that this is not how it should have been done and added,
Though I have a lot of faith in DRDO and India’s pharmaceuticals, but having said that, I believe, this is not how science works. You have to and have to do peer review of the findings, you have to tell, what are the targets and what is the drug or medicine is targeting to reduce – mortality, oxygen use or ventilator use. What are the outcomes we are looking at – this all should be in public domain and research domain. And that has not been done in this case. It was also done in the other vaccines for COVID-19 and that is why there was vaccine hesitancy earlier. I believe, the drug can also have complications and side-effects, but no-one is talking about that.
Further giving an example of the plasma therapy, which was continued in the country for the treatment of COVID-19 patients, Dr Ray added,
The way the research has changed for the treatment of COVID now is a point to worry. In the initial phase of the pandemic, the research was done differently and was very different from now what we are seeing. I think, now we are rushing into things and the same was the case with plasma therapy. Most of the doctors initially had said that plasma therapy will not work, but we did it, without even having strong evidence for it. The same should not happen with this drug.
Also Read: Coronavirus Explained: All You Need To Know About The COVID-19 Vaccine For Children In India
Reiterating the same point of lack of evidence in the public domain, Dr Yatin Mehta, Chairman, Medanta Critical Care said,
I think, we are getting too excited about the drug. It’s too early to do that. We should first follow the full protocol for releasing a drug, which include scientific paper and peer review studies, which has not be done in this case. I sincerely hope the drugs works, but I also think, trying it out on just 220 is a very small number.
On the contrary, Dr Dinesh Singh, Senior Director, Max Super Hospital, Vaishali who HAS had some experience in handling this drug in past for his patients says,
As far as my experience in handling this drug in patients with brain tumour and cancer, I have seen that if you give low dosage of this modified glucose to patients it is treated safe. Even in this trial, we are just giving 45 milligrams per kilogram body weight of the dosage.
Whereas, Dr Ray says that the evidence of this glucose working even in cancer patients is very less. He said,
Basically, this is a dummy glucose, which helps in slowing the process of cells or virus from multiplying. It sounds very good, but it has not worked in past, nor we have much data on the same to prove its efficacy. What we need to know for this new drug is data, which is currently not there at all, at least in the public domain. Why can’t the data for our vaccines be more transparent like FDA, the way they had put the data for Pfizer and Moderna vaccine, is something we should look at. Every single information for both these vaccines were put live on the public domain.
NDTV – Dettol Banega Swasth India campaign is an extension of the five-year-old Banega Swachh India initiative helmed by Campaign Ambassador Amitabh Bachchan. It aims to spread awareness about critical health issues facing the country. In wake of the current COVID-19 pandemic, the need for WASH (Water, Sanitation and Hygiene) is reaffirmed as handwashing is one of the ways to prevent Coronavirus infection and other diseases. The campaign highlights the importance of nutrition and healthcare for women and children to prevent maternal and child mortality, fight malnutrition, stunting, wasting, anaemia and disease prevention through vaccines. Importance of programmes like Public Distribution System (PDS), Mid-day Meal Scheme, POSHAN Abhiyan and the role of Aganwadis and ASHA workers are also covered. Only a Swachh or clean India where toilets are used and open defecation free (ODF) status achieved as part of the Swachh Bharat Abhiyan launched by Prime Minister Narendra Modi in 2014, can eradicate diseases like diahorrea and become a Swasth or healthy India. The campaign will continue to cover issues like air pollution, waste management, plastic ban, manual scavenging and sanitation workers and menstrual hygiene.
[corona_data_new]